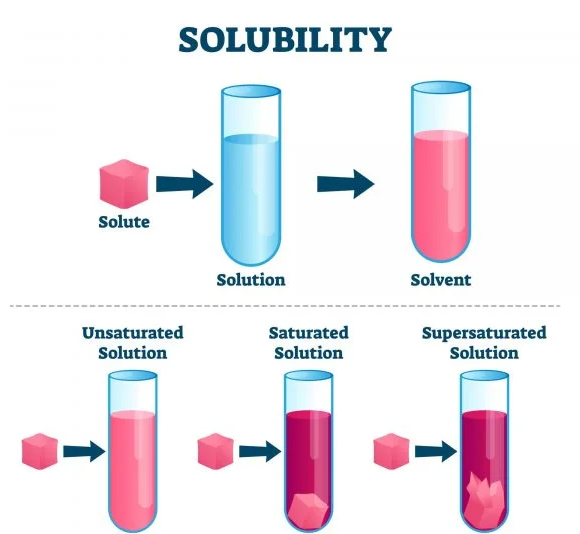
Solubility is a foundational concept in chemistry, referring to the maximum amount of a substance (solute) that can dissolve in a specific solvent at a given temperature and pressure, creating a homogeneous mixture. It’s essentially a measure of a substance’s willingness to dissolve and form a solution.
Understanding solubility involves recognizing the intermolecular interactions between the solute and solvent. When these interactions are favorable, solubility increases. Conversely, if the interactions are weak or nonexistent, solubility decreases. The interactions can vary from ionic bonds to hydrogen bonding or London dispersion forces, depending on the nature of the substances involved.
Factors Influencing Solubility
Various factors significantly influence solvency. Firstly, temperature plays a critical role. In general, solvency tends to increase with temperature for solid solutes, as higher temperatures provide more energy for the solute particles to break apart and mix with the solvent. However, for gases, solubility typically decreases with increasing temperature due to decreased gas solubility as the temperature rises.
Pressure also affects solubility, especially for gases. Henry’s Law dictates that the concentration of a gas in a liquid is directly proportional to the partial pressure of that gas above the liquid. Therefore, higher pressures lead to increased gas solubility.
Moreover, the nature of the solute and solvent molecular structure influences solubility. Polar substances tend to dissolve better in polar solvents due to the compatibility of their charges, while nonpolar substances dissolve better in nonpolar solvents. The size and shape of molecules also play a role, affecting how easily they can fit into the solvent’s structure.
The Significance of Solubility in Chemistry
Solvency is paramount in chemistry as it underpins various essential processes and applications. In chemical reactions, solubility impacts the reaction’s progress and yield. Reactants need to dissolve in a solvent to interact and form products. Understanding solubility aids chemists in predicting reactions and designing experiments for desired outcomes.
Furthermore, solubility is crucial in crystallization processes, where dissolved substances precipitate from a solution to form crystals. This process is fundamental in industries like pharmaceuticals, where pure compounds are needed for drug formulation.
In pharmaceuticals, solvency directly affects a drug’s bioavailability and efficacy. Formulators need to consider the solubility of the active ingredients to design medications that dissolve effectively and get absorbed by the body.
Real-world Applications of Solvency
Solubility has tangible applications in our daily lives. In cooking, understanding the solvency of different ingredients influences the flavors and textures of the final dish. For instance, the dissolution of sugar in water is a key aspect of many recipes.
In environmental science, solvency plays a role in understanding phenomena like groundwater contamination. Understanding which substances are soluble in water helps predict their dispersion and potential impact on the environment.
In the realm of industrial chemistry, solvency is crucial in processes like metal extraction and purification, where substances need to dissolve effectively for efficient processing.
Exploring Solubility in Mixtures
Solvency, a fundamental concept in chemistry, seamlessly extends its reach into mixtures, enriching our comprehension of chemical interactions. In mixtures, multiple substances can dissolve simultaneously in a solvent, creating intricate and dynamic compositions. This section will delve deeper into the world of mixtures and how solubility influences their behavior.
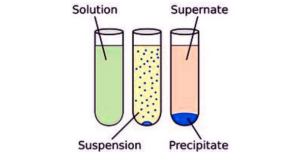
When different substances dissolve in a common solvent, they can interact in various ways. Some may form homogeneous solutions, where the molecules of the substances are evenly distributed. Others might engage in limited solubility, resulting in a saturated solution or even forming precipitates.
Understanding how substances interact and dissolve in each other within a mixture provides valuable insights across various applications. In pharmaceuticals, for instance, this knowledge is critical for creating effective drug combinations with optimal solubility and absorption rates.
Further Insights through Phase Diagrams and Solubility Curves
In the realm of mixtures, phase diagrams, and solvency curves serve as indispensable tools. Phase diagrams visually represent the equilibrium phases of a mixture under specific conditions of pressure and temperature.
Solvency curves within phase diagrams provide essential information about the solvency of components within the mixture at varying temperatures and pressures. Understanding these curves enables us to predict phase transitions, helping industries design and optimize processes efficiently.
For instance, in the production of alloys, knowledge of solvency curves is vital for achieving the desired material properties. By carefully considering temperature and pressure conditions, manufacturers can control the solubility of different elements in the alloy, ensuring the final product meets the required specifications.
Conclusion
In conclusion, solvency is a fundamental concept in chemistry, impacting reactions, crystallization, formulation of solutions, and various other processes. A thorough grasp of dissolvability is essential for both students and practitioners in the field of chemistry. It enables informed decision-making, efficient processes, and innovative applications that drive advancements in chemistry and related disciplines.