Iron(III) chloride, chemically represented as FeCl3, holds a pivotal position in the realm of chemistry. This article aims to comprehensively explore the intricacies of this compound, delving into its fundamental properties, chemical structure, and composition. By shedding light on these aspects, we endeavor to provide a thorough understanding of FeCl3 and its diverse applications.
Properties of Iron(III) Chloride
Iron(III) chloride exhibits several notable properties that define its behavior and applicability. Firstly, it exists primarily in a solid state, appearing as a crystalline substance. Notably, it possesses high hygroscopicity, meaning it readily absorbs moisture from the surrounding environment. This property is essential to consider when handling and storing FeCl3.
Another distinctive characteristic of FeCl3 is its color. In its solid state, it displays a characteristic reddish-brown hue, giving it a recognizable appearance. However, upon dissolving in water, it forms a yellow-brown solution due to the formation of [Fe(H2O)6]3+ ions.
Regarding solubility, FeCl3 exhibits varying degrees based on temperature and solvent properties. Generally, it is highly soluble in water, a property crucial to its applications, particularly in aqueous solutions used in various chemical processes.
Chemical Structure of Iron(III) Chloride
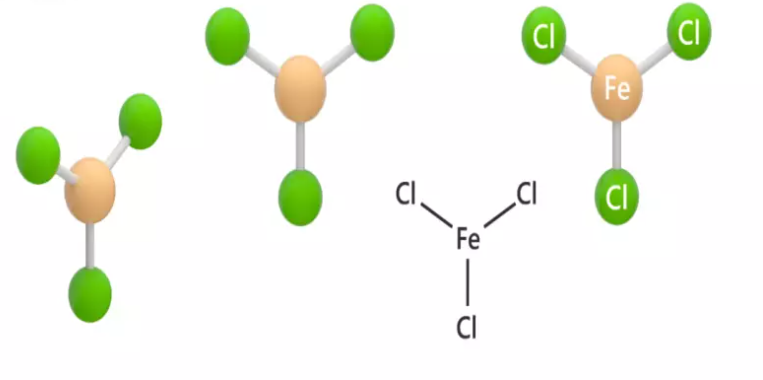
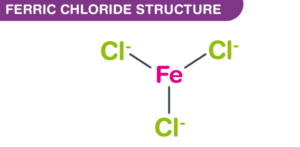
The chemical structure of FeCl3 is essential to understand its behavior and reactivity. Molecularly, it comprises one iron atom bonded to three chlorine atoms, following the formula FeCl3. This structure results in a trigonal planar geometry around the central iron atom, showcasing a covalent bond between iron and chlorine.
Beyond this covalent nature, Iron(III) chloride also showcases ionic characteristics, especially in its aqueous state. When dissolved in water, it dissociates into Fe3+ cations and Cl- anions, highlighting its versatility and the interplay between ionic and covalent bonding.
Composition of Iron(III) Chloride
The composition of Iron(III) chloride, as the name implies, consists of one iron atom bonded to three chlorine atoms. This forms a molecule with a molar mass of approximately 162.2 grams per mole. Understanding this composition is fundamental for stoichiometric calculations and comprehending its behavior in chemical reactions.
Applications and Uses of Iron(III) Chloride
The versatility of FeCl3 makes it indispensable across various applications. Industrially, it serves as a catalyst for numerous processes, particularly in organic synthesis. It facilitates reactions like the Friedel-Crafts acylation, crucial for synthesizing various organic compounds.
In the realm of water treatment, Iron(III) chloride plays a pivotal role. Its high solubility and ability to form complexes make it effective in removing impurities and contaminants from water, ensuring it meets the required standards for consumption.
FeCl3 is also widely employed in the electronics industry, primarily for etching printed circuit boards (PCBs). Its corrosive properties enable it to precisely remove the copper layers from PCBs, a critical step in their production.
Moreover, Iron(III) chloride is a key reagent in organic chemistry, facilitating critical transformations and enabling the synthesis of complex molecules. It finds applications in pharmaceuticals, dyes, and pigments.
Final Thoughts
In conclusion, comprehending the properties, chemical structure, and composition of Iron(III) chloride lays a strong foundation for understanding its diverse and extensive applications. As a catalyst, a water treatment agent, or a vital component in organic chemistry, FeCl3 remains an indispensable element contributing to advancements in chemistry and various industrial sectors.
Frequently Asked Questions (FAQs)
Is FeCl3 dangerous to handle due to its acidic nature?
Yes, FeCl3 is corrosive and has an acidic nature. Direct contact can cause skin irritation and burns. Proper handling, including wearing appropriate protective gear and following safety protocols, is essential when dealing with FeCl3 to ensure safety.
Can FeCl3 be used in drinking water treatment?
Yes, Iron(III) chloride is used in water treatment to remove impurities and contaminants. However, it’s crucial to use it in controlled amounts and ensure the treated water meets regulatory standards for safe consumption.
Can FeCl3 be used at home for any applications?
While FeCl3 has various applications, its corrosive and hazardous nature makes it unsuitable for general household use. It is primarily used in controlled industrial or laboratory settings with appropriate safety measures.
What are some common uses of FeCl3 in industrial applications?
FeCl3 is a catalyst in organic synthesis, aiding in reactions like the Friedel-Crafts acylation. It’s also used in etching PCBs in the electronics industry and as a coagulant in wastewater treatment processes.
How does FeCl3 function as a catalyst in chemical reactions?
FeCl3 is a Lewis acid catalyst, meaning it can accept an electron pair from another molecule during a reaction. This property enables it to facilitate specific chemical reactions, especially in organic synthesis, by altering the reaction pathway or speeding up the reaction.
What safety precautions should be taken when handling FeCl3?
When handling FeCl3, wear appropriate protective gear like gloves, goggles, and a lab coat. Work in a well-ventilated area to minimize inhalation exposure. In case of contact with skin or eyes, rinse immediately with plenty of water and seek medical attention.